Microalgae products: From novel food regulations to innovative, sustainable protein alternatives
Authors: Dario Dongo, Food and Agriculture Requirements (FARE), Marie-Christine Thurm, European Food Information Council (EUFIC)
What are microalgae and how can we use them?
Microalgae are tiny plant-like microorganisms that live in various aquatic environments. They are usually made from one single cell or a small number of cells which can quickly grow and multiply into a large biomass rich in nutrients. Microalgae come in different shapes and colours and can be used for different industrial purposes from food and feed to cosmetics and biofuel production. For example, some of the more well-known microalgae species include Spirulina and Chlorella and are used in food supplements.

Microalgae have great potential to be increasingly used as ingredients in sustainable and healthy food and feed products: With the right technology and processes, they can be produced in a very sustainable way, they have a high protein content, and also contain valuable nutrients such as phenolic compounds, vitamins and minerals. Over the last years, the appetite for alternative sources of proteins and functional ingredients has grown. Microalgae seem to be in a very good position, also because of decades of experience in cultivating them for different purposes. Although at EU level, consumers are less familiar with microalgae, at global level, there is a good consumer acceptance.
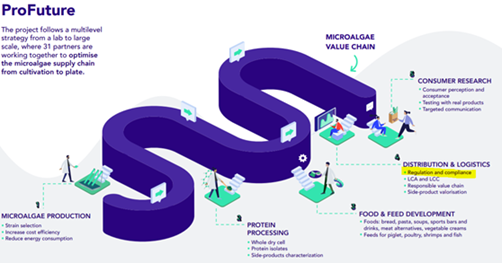
The EU funded ProFuture project scales up microalgae production, setting food safety as a priority
The ProFuture projects looks at the whole microalgae value chain – from the production to the consumption of microalgae products. This also includes regulation and compliance, defining if we can consume the different microalgae species and the developed products by assessing food security, food safety and sustainability. The aim: Protecting the consumer’s health and avoiding misleading information, while boosting the alternative protein market.
Some microalgae species are considered as Novel Food. What does that mean?
Food businesses are responsible to guarantee the safety of all food they place in the EU market. In case of microalgae, they need to properly identify its species and strains, which is not always an easy task. To then legitimately place microalgae in the market, they need to assess if the Novel Foods Regulation (EU) No 2015/2283 needs to be applied or not. When are foods defined as ‘novel’? If they have no proven track of safe consumption to a significant degree in the EU before 15 May 1997. This is when the first regulation on novel food came into force.
A recent article on the state of the art of microalgae in the EU lists microalgae that are not recognized as novel, species for which the authorization procedure is in progress and those are already approved as Novel Food (the article is currently available in Italian but will soon be translated into more languages).
As a next step, the food business who wants to introduce a Novel Food must apply for its preventive authorization at EU level. The application must show scientific evidence of the food safety for human consumption, and its intended uses. EFSA has published guidelines and has done workshops on the general principles that need to be followed to provide evidence of novel food safety. Obviously, there cannot be a ‘one-size-fits-all’ guidance for the enormous variety of products that may fall within the scope of the Novel Food Regulation. Nonetheless, scientific literature and precedent dossiers evaluations by EFSA offer a valuable benchmark on the priorities and the specific criteria to be followed. In ProFuture, the partner Food and Agriculture Requirements (FARE) provides the regulatory and technical advice that is needed to legitimately place microalgae in the European market. Other project partners take care of the safety assessment, for example carefully checking the developed products regarding heavy metals content, potential allergens or microbiological stability.

Further info on food safety of alternative proteins
You can learn more about the topic in a recording of a webinar on safety and regulatory challenges of alternative proteins. It took place in December 2021, organized by Horizon4Proteins,a collaboration between 4 EU projects working on alternative proteins for food and feed. Experts from the projects SUSINCHAIN and NEXTGEN Proteins talked about chemical safety of insects and regulatory challenges of alternative proteins.